-
Module 2.0 How to be Successful in this Course
-
Module 2.1 Introduction to Natural Gas
-
Module 2.2 The Natural Gas Industry in British Columbia
- Overview
- Learning Outcomes
- Natural Gas Science – The Simple Version
- Natural Gas Science – Chemistry
- Natural Gas Science – Physics
- Natural Gas Science – Units of Measurement
- Natural Gas Science – Geology
- Natural Gas Resources and Uses
- Oversight of the Natural Gas Industry
- Understanding Land Rights and Natural Gas
- Energy and the Future
-
Module 2.3 Upstream – Well Site Selection, Preparation and Drilling, Completion, Production, Water Recycling, and Reclamation
- Learning Outcomes
- The Upstream Sector – Extraction and Processing
- The Upstream Sector – Exploration and Site Selection
- The Upstream Sector – Preparation and Drilling
- The Upstream Sector – Completion
- The Upstream Sector – Production
- The Upstream Sector – Water Recycling
- The Upstream Sector – Reclamation
- Upstream Companies and Jobs in British Columbia – Companies
- Upstream Companies and Jobs in British Columbia – Industry Associations
- Upstream Companies and Jobs in British Columbia – Professional Associations
- New Vocabulary
-
Module 2.4 Midstream – Transportation, Processing, Refining
- Learning Outcomes
- The Midstream Sector
- The Midstream Sector – Processing Natural Gas
- The Midstream Sector – Liquefied Natural Gas
- The Midstream Sector – An Emerging Industry
- The Midstream Sector – Processing LNG
- The Midstream Sector – Proposed LNG Projects in British Columbia
- Transportation
- Midstream Companies and Jobs in British Columbia
-
Module 2.5 Downstream – Refining and Markets
-
Module 2.6 Health and Wellness in the Natural Gas Industry
-
Module 2.7 Safety
-
Module 2.8 Terminology and Communication
-
Module 2.9 Jobs and Careers
- Learning Outcomes
- Industry Outlook
- Technology is Changing Workforce and Skills
- Employment in the Natural Gas Industry
- Employment in the Natural Gas Industry – Types of Employment
- Employment in the Natural Gas Industry – Range of Jobs
- Employment in the Natural Gas Industry – High Demand Jobs and Occupations
- Occupational Education and Training
-
Module 3.0 How to be a Valued Employee
-
Module 3.1 Identifying Interests and Skills
-
Module 3.2 Looking for Employment in Natural Gas
-
Module 3.3 Applying for Employment in Natural Gas
Here are some key chemistry terms and concepts that are helpful to understand when working with natural gas.
Matter and substance

Anything that occupies space and has mass (weight) is matter. On earth, matter is found in three states: solid, liquid, or gas. Substance is matter who’s physical and chemical properties are the same throughout (homogenous); substances can change from one state of matter to another.
Atoms and molecules
An atom is the smallest particle into which a substance can be physically separated. Molecules are formed when two or more atoms chemically bind together.
Elements and compounds
Elements are substances which contain only one kind of atom. There are three types of elements: metals, non-metals, and semimetals; most elements are metals. Every element is represented by a unique symbol. These symbols are organized through what is referred to as a periodic table. The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements.
Compound substances are formed by a chemical process combining two or more elements. Compounds are defined by their chemical formulas. For example:
- Oxygen or O2—Oxygen is formed when two oxygen atoms bind together; the “O” refers to the oxygen atom, and the “2” refers to the number of oxygen atoms bonded to one another.
- Air contains about 20.8% oxygen.
- Nitrogen or N2—Nitrogen is formed when two nitrogen atoms bind together; the “N” refers to the nitrogen atom, and the “2” refers to the number of nitrogen atoms bonded to one another.
- Air contains about 78.1% oxygen.
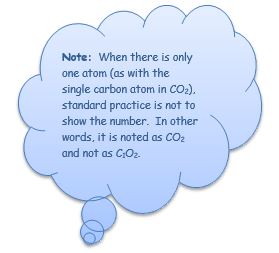 Carbon Dioxide or CO2— Carbon Dioxide is formed when one carbon atom binds with two oxygen atoms; the “C” refers to the single carbon atom, the O refers to oxygen, with the “2” referring to the number of oxygen atoms.
Carbon Dioxide or CO2— Carbon Dioxide is formed when one carbon atom binds with two oxygen atoms; the “C” refers to the single carbon atom, the O refers to oxygen, with the “2” referring to the number of oxygen atoms.
- Air contains about 0.04% CO2.
- Hydrocarbons—hydrocarbons are organic compounds formed by single bonds between hydrogen and carbon atoms; when a carbon atom is surrounded by hydrogen atoms, it is referred to as a saturated hydrocarbon, and alkanes are a type of saturated hydrocarbons that are flammable;
the formula for alkanes is CnH2n+2. These are the hydrocarbons that make up natural gas.
Putting it all together
- Natural gas is a compound hydrocarbon substance made up of different alkanes
- Table 1 shows the formal annotation for the key hydrocarbons or alkanes in natural gas.
Table 1: Hydrocarbons and Alkanes in Natural Gas (CNH2N+2)
| Number of Carbon Atoms |
Number of Hydrogen Atoms |
Formula | Common Name |
|---|---|---|---|
| 1 | 4 | CH4 | Methane |
| 2 | 6 | C2H6 | Ethanee |
| 3 | 8 | C3H8 | Propane |
| 4 | 10 | C4H10 | Butane |
| 5 | 12 | C5H12 | Pentane |
| 6 | 14 | C6H14 | Hexane |
| 7 | 16 | C7H16 | Heptane |
| 8 | 18 | C8H18 | Octane |
| 9 | 20 | C9H20 | Nonane |
| 10 | 22 | C5H22 | Decane |
The most abundant alkane in natural gas is methane. The terms gas, natural gas, and methane are often used interchangeably.
- Dry gas is almost pure methane; wet gas has more of the other hydrocarbons
- Methane, ethane, propane, and butane are gases used directly as fuels
- Pentane, Hexane and Heptane make up what is known as condensate
- Gasoline is a mixture of alkanes from pentane up to decane
- Kerosene contains alkanes with 10 to 16 carbon atoms per molecule
- Alkanes with 17 or more carbon atoms per molecule are solids at room temperature, such as petroleum jelly, paraffin wax, and asphalt.
